Learn The Science
Cerebrolysin: The NūūtroSmart Solution
Treatment of neurological disorders with Cerebrolysin helps to increase the quality of life for patients.
The efficacy of Cerebrolysin has been proven in 87 double-blind studies and trials with more than 17,000 patients.
Cerebrolysin
Neuroprotection & Neurorecovery
References
1. Wronski R et al., Inhibitory effect of a brain derived peptide preparation on the intracellular Calcium Ca++- dependent protease, calpain. J Neural Trans 2000; 107: 145-157
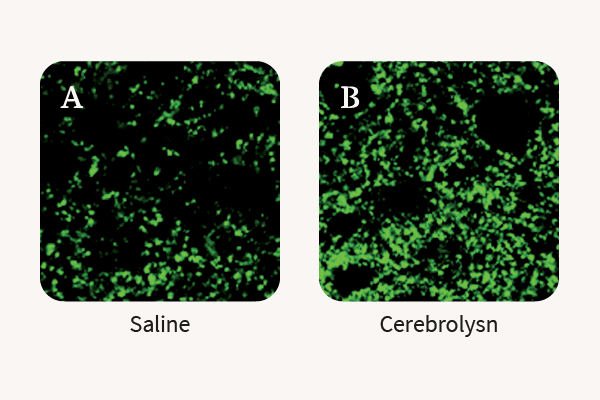
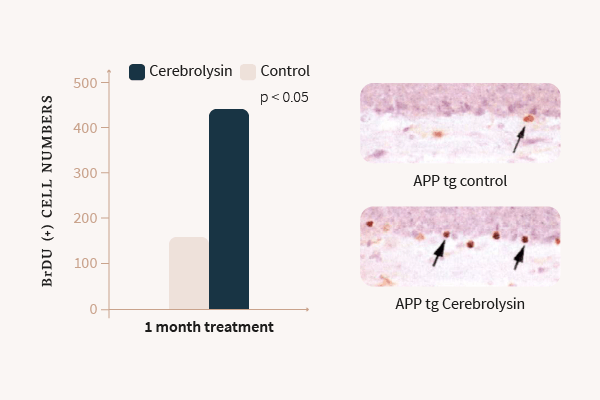
2. Rockenstein E. et al., Effects of Cerebrolysin on neurogenesis in an APP transgenic model of Alzheimer’s disease, Acta Neuropathol 2007; 113; 265-275
3. Alvarez X.A. et al., Cerebrolysin reduces microglial activation in vivo and in vitro: a potential mechanism of neuro-protection, J Neuronal Transm 2000; 59; 281- 292
4. Sugita Y., Kondo T Kanazawa A, Itou T., Mizuno Y (1993): Protective effect of FPF 1070 (Cerebrolysin) on delayed neuronal death in the gerbil – detection of hydroxyl radicals with salicylic acid. No To Shinkei; 45/4; 325-331
5. Hutter-Paier B. et al., Death of cultured telencephalon neurons induced by glutamate is reduced by the peptide derivate Cerebrolysin; J Neural Transm 1996; 47; 267-273
6. Rockenstein E. et al., The neuroprotective effects of Cerebrolysin trade mark in a transgenic model of Alzheimer’s disease are associated with improved behavioral performance. J Neural Transm 2003; 110; 1313-27
7. Bornstein, Natan M., et al. „Safety and efficacy of Cerebrolysin in early post-stroke recovery: a meta-analysis of nine randomized clinical trials.“ Neurological Sciences 39.4 (2018): 629-640.
8. Ladurner, G.; Kalvach, P.; Mössler, H. Neuroprotective treatment with cerebrolysin in patients with acute stroke: a randomised controlled trial. Journal of neural transmission, 2005, 112. Jg., Nr. 3, S. 415-428.
9. Lang, Wilfried, et al. A prospective, randomized, placebo‐controlled, double‐blind trial about safety and efficacy of combined treatment with alteplase (rt‐PA) and Cerebrolysin in acute ischaemic hemispheric stroke. International Journal of Stroke, 2013, 8. Jg., Nr. 2, S. 95-104.
10. Muresanu, Dafin F., et al. Cerebrolysin and recovery after stroke (CARS): a randomized, placebo-controlled, double-blind, multicenter trial. Stroke, 2016, 47. Jg., Nr. 1, S. 151-159.
11. Chang, Won Hyuk, et al. Cerebrolysin combined with rehabilitation promotes motor recovery in patients with severe motor impairment after stroke. BMC neurology, 2016, 16. Jg., Nr. 1, S. 31.
12. Heiss, Wolf-Dieter, et al. Cerebrolysin in patients with acute ischemic stroke in Asia: results of a double-blind, placebo-controlled randomized trial. Stroke, 2012, 43. Jg., Nr. 3, S. 630-636.
13. Vester, Johannes C., et al. „Cerebrolysin after moderate to severe traumatic brain injury: prospective meta-analysis of the CAPTAIN trial series.“ Neurological Sciences (2021): 1-11.
14. König, P.; Waanders, R.; Witzmann, A. Cerebrolysin in TBI: a pilot study of a neurotropic and neurogenic agent in the treatment of acute traumatic brain injury. Journal Für Neurologie Neurochirurgie Und Psychiatrie, 2006, 7. Jg., Nr. 3, S. 12-20.
15. Muresanu, Dafin F., et al. „Efficacy and safety of cerebrolysin in neurorecovery after moderate-severe traumatic brain injury: results from the CAPTAIN II trial.“ Neurological Sciences 41.5 (2020): 1171-1181.
16. Chaisoonthon, Pipat. Traumatic brain injury-treatment with cerebrolysin. Journal of Sakon Nakhon Hospital, 14. Jg., Nr. 2.,2011
17. Chen, Chun-Chung, et al. Cerebrolysin enhances cognitive recovery of mild traumatic brain injury patients: double-blind, placebo-controlled, randomized study. British journal of neurosurgery, 2013, 27. Jg., Nr. 6, S. 803-807.
18. Gauthier, Serge, et al. Cerebrolysin in mild-to-moderate Alzheimer’s disease: a meta-analysis of randomized controlled clinical trials. Dementia and geriatric cognitive disorders, 2015, 39. Jg., Nr. 5-6, S. 332-347
19. Xiao, S.; Yan, H.; Yao, P. The efficacy of cerebrolysin in patients with vascular dementia: Results of a Chinese multicentre, randomised, double-blind, placebocontrolled trial. Hong Kong Journal of Psychiatry, 1999, 9. Jg., Nr. 2, S. 13.
20. Guekht, Alla B., et al. Cerebrolysin in vascular dementia: improvement of clinical outcome in a randomized, double-blind, placebo-controlled multicenter trial. Journal of Stroke and Cerebrovascular Diseases, 2011,
21. Jg., Nr. 4, S. 310-318 21. Chen, Ning, et al. Cerebrolysin for vascular dementia. Cochrane Database Syst. Rev, 2013, 1. Jg.
22. Ruether, E., et al. Sustained improvements in patients with dementia of Alzheimer’s type (DAT) 6 months after termination of Cerebrolysin therapy. Journal of neural transmission, 2000, 107. Jg., Nr. 7, S. 815-829.
23. Panisset, M., et al. Cerebrolysin in Alzheimer’s disease: a randomized, double-blind, placebo-controlled trial with a neurotrophic agent. Journal of neural transmission, 2002, 109. Jg., Nr. 7-8, S. 1089-1104.
24. Alvarez, X. A., et al. A 24‐week, double‐blind, placebo‐controlled study of three dosages of Cerebrolysin in patients with mild to moderate Alzheimer’s disease. European journal of neurology, 2006, 13. Jg., Nr. 1, S. 43-54.
25. Ruether, E., et al. A 28-week, double-blind, placebo-controlled study with Cerebrolysin in patients with mild to moderate Alzheimer’s disease. International clinical psychopharmacology, 2001, 16. Jg., Nr. 5, S. 253-263
The NūūtroSmart Solution
Cerebrolysin Protocols
Intravenous Cerebrolysin Infusions
We have 5ml, 10ml and up to 50ml intravenous infusions of Cerebrolysin available by appointment at the clinic.
Administered by trained and licensed medical professionals.
At-Home Subcutanous Injections
We can also complement our intravenous Cerebrolysin infusions with at-home subcutaneous injections.
If you prefer to complete an at-home course of Cerebrolysin, we can assist with that as well.
Jessica (BSc Biomedicine, MSc Longevity, CHP) our Cerebrolysin expert.
Nūūtro
Quality You Can Trust, Expertise You Can Rely On.
The Nūūtro Assurance: Quality Above All
When we say we know our supplier, we mean it.
We have seen firsthand the meticulous care that goes into every batch of peptides.
From the precision of ingredient selection to the exacting quality control measures, we ensure that every product that reaches your hands is the best it can be.
This level of direct oversight is what sets Nūūtro apart.
It’s the reason why our peptides come at a price point that reflects their true value.
High-quality ingredients and a robust, transparent production process aren’t cheap, but they are the cornerstone of effective and safe peptide therapy.
Enquire Today
To learn more about our Cerebrolysin IV Therapy, please request an appointment at info@nuutro.co.uk